AN0105: Human IFN-α (subtype 2) ELISA with Optimiser™ Microplates
Assay Solution for Mabtech ELISA for Human Interferon-alpha (IFN-α) (subtype 2) (Product Code: 3423-1H-6)
Victor Moore; Applied Science Group, MiCo BioMed, Cincinnati, OH USA
Introduction:
MiCo BioMed’ Optimiser™-based ELISAs offer a rapid, sensitive, and specific chemifluorescent-based ELISA procedure for the measurement of analytes using very small sample volumes. The Optimiser™ plate is SBS/ANSI-compliant and is compatible with traditional 96-well microplate instrumentation.
A Human (Hu) IFN-α (subtype 2) ELISA utilizing reagents from Mabtech (Product Code 3423-1H-6) has been successfully transferred from a conventional 96-well ELISA plate format to an Optimiser™ microplate platform to achieve the following key performance benefits.
|
Optimiser™ Method Development Process:
Please refer to the Assay Transfer Guide (Technical Support section of MiCo BioMed’s website) for details on the method development process. Each process step listed below requires 1 microplate (conventional for Step 1) and represents 1 experiment. In Step 2, the optimal coating buffer is selected from a panel of 12 coating buffers developed by MiCo BioMed.
| Step 1: | Run a conventional ELISA to verify that the assay reagents perform as stated by the vendor(s). The human IFN-α (subtype 2) reagents were tested in a high-binding NUNC plate according to the protocol provided by the vendor. The operating range was confirmed as 4 – 400 pg/ml as stated by vendor. |
| Step 2: | Select the optimal coating buffer for use with the Optimiser™-based ELISA. The coating buffer screening test showed that OptiBind™-L allows for most efficient binding of capture antibody to microfluidic channel surface. |
| Step 3: | Run antibody titrations to select the optimal capture and detection antibody concentrations. The titration study shows that 4 µg/ml of capture and 2 µg/ml of detection antibody exhibit best performance. As described further, this Optimiser™-based ELISA is more sensitive than a conventional plate ELISA and the user should determine the optimal antibody concentrations to maximize savings and achieve the desired operating range. |
Note that while capture and detection antibody concentrations for Optimiser™-based ELISAs may be 2 -4 times greater than those used in conventional ELISAs; the total quantity of antibody used in Optimiser™-based methods is significantly lower than that used in conventional ELISAs due to the much smaller reagent volumes used in the Optimiser™-based methods. Furthermore, sensitivity can be readily “tuned” for Optimiser™-based ELISAs using the repeat loading approach and antibody use can be further minimized if desired.
|
Comparison of Antibody Requirements for Conventional and Optimiser™-based ELISAs. |
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
Optimiser™-Based ELISA and Results for Human IFN-α (subtype 2):
The detailed procedure for this Optimiser™-based ELISA is contained in a companion document (User Manual or Detailed Experimental Protocol). The materials used in the procedure are specified in the Materials Table.
|
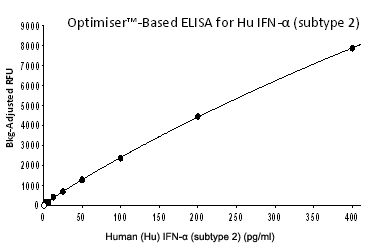 |
The plate is read using an FLx800™ Fluorescence Microplate Reader (BioTek Instruments, Inc.) equipped with a 528/20 nm excitation filter and a 590/35 nm emission filter with a sensitivity setting of 44. The data is analyzed using Gen5™ software (BioTek Instruments, Inc.) and a standard curve is created by plotting human IFN-α (subtype 2) concentration vs background-adjusted RFU using a 4-parameter curve fit.
|
Materials Used:
| Material | Vendor | Vendor’s Catalog # | Vendor Contact |
| ELISA for Human IFN-α (subtype 2) | Mabtech | 3423-1H-61 | 1-513-871-4500 |
| NUNC Maxisorp microplate | Thermo Scientific | 456537 | 1-800-625-4327 |
| Optimiser™ microplate (with Holder) 2 | MiCo BioMed | OPH-10 |
www.micobiomed-usa.com |
| Polypropylene v-bottom plate | OPT/FL-231 | ||
| Coat buffer test panel 3 | OMR-TEST | ||
| Buffer reagent pack (with substrate) 2 | OMR-10-J | ||
| Streptavidin-HRP for Optimiser™ | OMR-HRP |
| 1 Contains capture and detection antibodies, rHu IFN-α (subtype 2), and SAv-HRP. |
| 2 Optimiser™ plates and corresponding OptiMax™ buffer reagents are also available in 2-plate and 50-plate configuration. |
| 3 OMR-TEST is required only for assay transfer.
FOR RESEARCH USE ONLY. Not for use in diagnostic procedures. |

